Sodium In Water = Explosion
Traditionally, students learn in general chemistry that when a piece of sodium metal is placed in a beaker filled with water, the following occurs -- which is shown in the video below from 'YouTube':
There is a whole lot of complex chemistry happening inside the beaker in the video above. Why? Because the dynamics of the reaction are shaped by multiple factors: shape of metal, changing surface charge, heat generated, and fluid dynamics -- to name a few.
New Video Of Sodium In Water
Recently, an article appeared on the website "ChemistryWorld.com" titled "Tamed Sodium In Water Comes Out Of The Blue" in which a new high speed video recording shows in detail more chemical information about the reaction of sodium in water. Here is the video below:
In the first video, the description is given by the authors in the current article as the following:
Philip Mason and Pavel Jungwirth of the Academy of Sciences of the Czech Republic in Prague and colleagues in Germany have done just that. They explain that under normal conditions, as soon as sodium touches water, electrons flow rapidly from the alkali metal to the water, forming hydrogen and hydroxide in a strongly exothermic process. In earlier work they showed that the outpouring of electrons leads to an enormous positive charge on the piece of metal, which makes it so electrostatically unstable that metal spikes shoot into the water, raising the surface area of metal in contact with water and accelerating the reaction in a so-called Coloumb explosion.
The chemical reaction which is described in the paragraph above can be written as the following:
In the reaction above, sodium metal (denoted by Na) reacts with water (H20) to form sodium hydroxide (NaOH) and hydrogen gas (H2). Note: Both "2"'s in the previous sentence should be subscript as shown in the reaction equation above. After looking at the reaction equation above along with the two videos, the following questions naturally arise:
How is the first video different than the second video?
Is the camera speed different?
How are the thermodynamics of the reaction "controlled"?
Here is the description by the author of the article mentioned above:
The team has now found a way to take control of this instability in a sodium–potassium alloy by adding a small amount of hexanol or by gently placing the alloy drop on water under an argon atmosphere. Held temporarily in this non-exploding state, the team could use a combination of high-speed camera imaging and visible and infrared spectroscopy to observe and characterize the system through each stage of the reaction.The alloy’s low density and the generation of gas buoys the drop and reduces interactions with water while continuous flushing with argon precluded ignition of the hydrogen. The team were able to observe the characteristic blue color of free electrons in solution – solvated electrons – with the naked eye. The slow motion film reveals that the energy-releasing reaction proceeds steadily, with the metal drop glowing red hot and eventually evaporating. The final product is a perfectly transparent drop of molten hydroxide that is briefly stabilized on the water as a result of the Leidenfrost effect, the same effect that lets water droplets bounce on a hot stove. The hydroxide droplet ends its life by whirling around the vessel for a few seconds and ultimately bursts spectacularly as it cools.
As I mentioned above, there was a whole lot of complex chemistry going on in the beaker above. To be able to slow the evolution of the hydrogen gas down in order to view (in greater detail) the difference in charges as the electrons go into solution is absolutely amazing. Mind blowing!
The researchers did a great job of discovering how to slow down the dynamics of the potentially explosive reaction. By changing the following parameters: drop height, surface reactivity, and fuel consumption rate. Lets briefly go over the differences of the two videos.
In order to control the dynamics (i.e., control the explosion), the researchers found in the study that the rate of reaction figured in the 'drop height.' Meaning, if the researchers had just dropped a chunk of metal into the beaker of water, the reaction would have been a much more vigorous reaction (more explosive). This suggests that the drop height adds an energetic factor to the rate of reaction.
The shape of the metal along with the surface made a contribution to the rate of reaction too. In the current study, the researchers chose to place a "spherical drop" of liquid alloy (alloy is a mixture of two metals) onto the surface of the water. Additionally, the surface of the water had a small layer of hexanol to slow the reactivity down further. This realization is important toward future research on alloys and explosion dynamics. Furthermore, the placement of the drop along with the hexanol reduced the evolution of hydrogen gas on the surface.
Typically, as seen in video #1, the metal is placed on the surface and the evolution of hydrogen gas acts like an additional fuel to generate either heat or an explosion. The researchers reduced the evolution of hydrogen gas by blowing 'Argon' gas across the surface. Argon gas is inert -- meaning -- the gas will not react. Inert gases are ideal to use when heating or mixing explosive materials in a closed container. The absence of oxygen reduced the chance of ignition.
Conclusion...
The results above show the impressive advance that high speed cameras offer to research chemists. Furthermore, the ability to study the reaction dynamics in greater detail help researchers explain each step in greater detail than was previously possible. Just think, 10 years ago, this was not possible to explain due to the inability to capture the video on camera. Technology is absolutely amazing.
The ability to study controlled explosions offer greater advances in the future of manufacturing munition and fuels. Anytime that a research scientist has the opportunity to study a reaction in much-much-much greater detail, he/she should take the opportunity to do so. As we study videos such as those above, we learn how to better understand the reactivity that is occurring inside the beaker. Explosion dynamics are difficult to study do to the inherent instability of the reactants and the fast pace of the reaction. Furthermore, there exists a huge safety factor to be concerned with. Although, having the ability to slow down the reaction by understanding the parameters which give rise to the explosive properties brings us one step closer to understanding explosion chemistry in greater detail. The results further validate our current understanding of science and help drive us into the future.
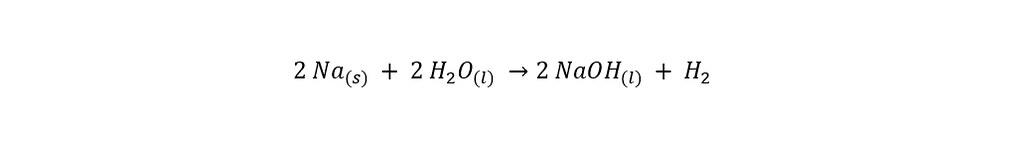
No comments:
Post a Comment